
Joannah Whitney of Greenfield wins 33rd annual Poet’s Seat Poetry Contest
GREENFIELD — After a few years submitting her work to the annual Poet’s Seat Poetry Contest, Greenfield poet and freelance writer Joannah Whitney placed first among the contest’s adult finalists Tuesday night at the Greenfield Public Library. The...

Is Steam Mill Road in Deerfield a public way? Land Court to decide as trial begins
GREENFIELD — The long-running saga of seeking to determine the status of Steam Mill Road in Deerfield has reached the courtroom, as the 2021 lawsuit began its bench trial at the Franklin County Justice Center on Wednesday, with opening statements and...
Sports

Country Club of Greenfield holding Youth Golf Camp June 24-27
The Country Club of Greenfield is holding its Youth Golf Camp from June 24-27. The camp will be capped at 25 children on a first come, first serve basis. The fee is $170 for the first family member and $140 for each additional family member. The camp...
 High schools: Lilly Ross records 100th career hit in Franklin Tech’s win over Northampton
High schools: Lilly Ross records 100th career hit in Franklin Tech’s win over Northampton
 Baseball: Wyatt Edes’ walk-off single lifts Frontier past Amherst 6-5
Baseball: Wyatt Edes’ walk-off single lifts Frontier past Amherst 6-5
 On The Ridge with Joe Judd: What time should you turkey hunt?
On The Ridge with Joe Judd: What time should you turkey hunt?
 High Schools: Jakhia Williams propels Turners girls track past Athol
High Schools: Jakhia Williams propels Turners girls track past Athol
Opinion

My Turn: April is second chance month
April is Second Chance Month, a time to focus on how our community can support our neighbors in central and western Massachusetts returning from incarceration.As attorneys with Community Legal Aid’s CORI/Re-Entry Unit, we see the life-changing power...
 My Turn: Judgmental about malaise
My Turn: Judgmental about malaise
 Ahmad Esfahani: Aiding and abetting and middle school angst
Ahmad Esfahani: Aiding and abetting and middle school angst
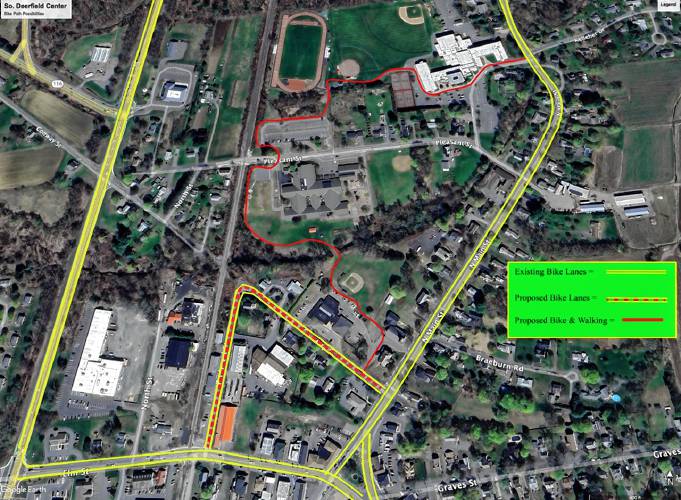 Greg Franceschi: Support bike lanes and walking paths in South Deerfield
Greg Franceschi: Support bike lanes and walking paths in South Deerfield
 Todd Damon: Liking the positive vibe of Greenfield lately
Todd Damon: Liking the positive vibe of Greenfield lately

Business

New Realtor Association CEO looks to work collaboratively to maximize housing options
SPRINGFIELD — As the Realtor Association of Pioneer Valley’s new CEO arrives to a Massachusetts housing market plagued by high prices and a lack of stock, he aims to work alongside elected officials to maximize the availability of different kinds of...
 New owners look to build on Thomas Memorial Golf & Country Club’s strengths
New owners look to build on Thomas Memorial Golf & Country Club’s strengths
 Cleary Jewelers plans to retain shop at former Wilson’s building until 2029
Cleary Jewelers plans to retain shop at former Wilson’s building until 2029
 Tea Guys of Whately owes $2M for breach of contract, judge rules
Tea Guys of Whately owes $2M for breach of contract, judge rules
 Primo Restaurant & Pizzeria in South Deerfield under new ownership
Primo Restaurant & Pizzeria in South Deerfield under new ownership
Arts & Life

Rescuing food and feeding people: Rachel’s Table programs continue to expand throughout western Mass
My great-grandmother’s oak dining table has graced my kitchen ever since my parents built the house in the 1980s. Looking at it makes me happy. The table — actually, almost any table — signifies history, nourishment, family and community.The...
Obituaries
 Constance Julia Dargis
Constance Julia Dargis
Constance Julia (LaMountain) Dargis Montague Center, MA - Constance "Connie" Julia Dargis, 98, formerly of Turners Falls MA, suffered an unexpected illness on November 13th and passed away peacefully on November 16, 2023 at the home of her ... remainder of obit for Constance Julia Dargis
 John H. Stevens Jr.
John H. Stevens Jr.
John H. Stevens, Jr. Gill, MA - John H. Stevens, Jr., 69, of Main Road died Monday 4/22/24 at home. He was born in Greenfield on December 9, 1954, one of nine children of John H. and Evelyn (Bassett) Stevens. John worked as a papermaker... remainder of obit for John H. Stevens Jr.
 Maryjean DelRosso
Maryjean DelRosso
Heath, MA - Maryjean (Sullivan) DelRosso passed away peacefully on April 19, 2024 at Baystate Medical Center in Springfield after a long illness. She is survived by her devoted husband, Joseph DelRosso. Maryjean is also survived by thr... remainder of obit for Maryjean DelRosso
 Carol Butler Watelet
Carol Butler Watelet
Greenfield, MA - Carol (Virginia) Soucie Butler Watelet, 85, passed away suddenly and peacefully on April 20, 2024, joining the love of her life, Mr. Wonderful, Robert Watelet who passed away 2 years earlier. Carol was born on March 17,... remainder of obit for Carol Butler Watelet

 Erving rejects trade school’s incomplete proposal for mill reuse
Erving rejects trade school’s incomplete proposal for mill reuse
 Parents question handling of threat at Erving Elementary School
Parents question handling of threat at Erving Elementary School
 Guest columnists Ellen Attaliades and Lynn Ireland: Housing crisis is fueling the human services crisis
Guest columnists Ellen Attaliades and Lynn Ireland: Housing crisis is fueling the human services crisis
 William Strickland, a longtime civil rights activist, scholar and friend of Malcolm X, has died
William Strickland, a longtime civil rights activist, scholar and friend of Malcolm X, has died
 Shutesbury voters to decide on battery storage, lighting bylaws
Shutesbury voters to decide on battery storage, lighting bylaws
 Healey weighs in on campus safety, protests
Healey weighs in on campus safety, protests
 Man allegedly steals $100K worth of items from Northampton, South Deerfield businesses
Man allegedly steals $100K worth of items from Northampton, South Deerfield businesses
 My Turn: Still No. 1 in male mass shootings 25 years after Columbine
My Turn: Still No. 1 in male mass shootings 25 years after Columbine
 Building conversion, battery storage bylaws up for vote at Sunderland Town Meeting
Building conversion, battery storage bylaws up for vote at Sunderland Town Meeting
 Sounds Local: A rock circus returns to Turners Falls: The Slambovian Circus of Dreams brings the fun Friday night at the Shea
Sounds Local: A rock circus returns to Turners Falls: The Slambovian Circus of Dreams brings the fun Friday night at the Shea Monroe election sees no contests, some positions without candidates
Monroe election sees no contests, some positions without candidates PHOTO: Flowing falls
PHOTO: Flowing falls PHOTO: A valuable simulation
PHOTO: A valuable simulation A day to commune with nature: Western Mass Herbal Symposium will be held May 11 in Montague
A day to commune with nature: Western Mass Herbal Symposium will be held May 11 in Montague Speaking of Nature: ‘Those sound like chickens’: Wood frogs and spring peepers are back — and loud as ever
Speaking of Nature: ‘Those sound like chickens’: Wood frogs and spring peepers are back — and loud as ever Hitting the ceramic circuit: Asparagus Valley Pottery Trail turns 20 years old, April 27-28
Hitting the ceramic circuit: Asparagus Valley Pottery Trail turns 20 years old, April 27-28 Best Bites: A familiar feast: The Passover Seder traditions and tastes my family holds dear
Best Bites: A familiar feast: The Passover Seder traditions and tastes my family holds dear
