Latest News
 Beacon Hill Roll Call: April 8 to April 12, 2024
Beacon Hill Roll Call: April 8 to April 12, 2024
 My Turn: Saving planet Greenfield
My Turn: Saving planet Greenfield

$50K allocated for Poet’s Seat Tower sandblasting as officials mull vandalism prevention
GREENFIELD — Following an affirmative vote by City Council this week, $50,000 will go toward sandblasting graffiti off of Poet’s Seat Tower, where vandalism has proven to be a persistent problem.Department of Public Works Director Marlo Warner II said...
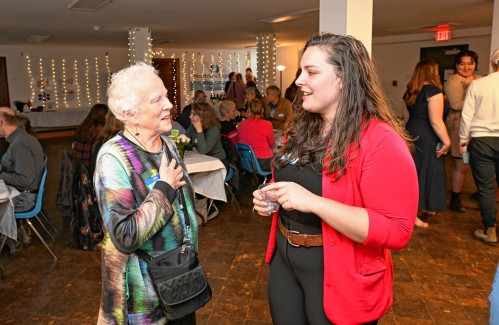
PHOTOS: Convening for a good cause
Most Read
 New owners look to build on Thomas Memorial Golf & Country Club’s strengths
New owners look to build on Thomas Memorial Golf & Country Club’s strengths
 Survey shows Northfield residents want new development — but not near their homes
Survey shows Northfield residents want new development — but not near their homes
 Orange man gets 12 to 14 years for child rape
Orange man gets 12 to 14 years for child rape
 As emergency action plan is crafted, Tree House to maintain 1,500 capacity for summer
As emergency action plan is crafted, Tree House to maintain 1,500 capacity for summer
 Real Estate Transactions: April 19, 2024
Real Estate Transactions: April 19, 2024
 Man granted parole for role in 2001 deaths of 2 Dartmouth College professors
Man granted parole for role in 2001 deaths of 2 Dartmouth College professors
Editors Picks
 Painting a more complete picture: ‘Unnamed Figures’ highlights Black presence and absence in early American history
Painting a more complete picture: ‘Unnamed Figures’ highlights Black presence and absence in early American history
 PHOTO: Double eagle
PHOTO: Double eagle
 North County Notebook: April 20, 2024
North County Notebook: April 20, 2024
 Best Bites: A familiar feast: The Passover Seder traditions and tastes my family holds dear
Best Bites: A familiar feast: The Passover Seder traditions and tastes my family holds dear
Sports

High schools: South Hadley softball holds off Frontier rally for 5-4 victory (PHOTOS)
A day after completing a comeback victory over Greenfield, the Frontier softball team looked like it had another rally in the works on Friday against South Hadley.The Tigers held a 1-0 lead going into the sixth inning when the Redhawks rallied,...
Opinion

Jessica Zhang: Weigh your choices — Solar power a better alternative
In his recent letter [“‘No’ means higher energy prices,” Recorder, March 26] Jim Bates is right that fossil fuels have powered societies across the globe — nobody disputes that. It’s the reality of pollution and carbon buildup in the atmosphere that...
 Gary Seldon: Solar Roller Earth Day River Ride
Gary Seldon: Solar Roller Earth Day River Ride
 Columnist Johanna Neumann: Reaping the rewards of rooftop solar
Columnist Johanna Neumann: Reaping the rewards of rooftop solar
 My Turn: Let’s leave miracle of trees well enough alone
My Turn: Let’s leave miracle of trees well enough alone

Business

New owners look to build on Thomas Memorial Golf & Country Club’s strengths
TURNERS FALLS — Members of the Snow family have added another golf course to their portfolio and have already begun work on cosmetic improvements.Kyle and Kelly Snow, along with Kyle’s parents Edward Snow Jr. and Kerrilynn Snow, bought the Thomas...
 Cleary Jewelers plans to retain shop at former Wilson’s building until 2029
Cleary Jewelers plans to retain shop at former Wilson’s building until 2029
 Tea Guys of Whately owes $2M for breach of contract, judge rules
Tea Guys of Whately owes $2M for breach of contract, judge rules
 Primo Restaurant & Pizzeria in South Deerfield under new ownership
Primo Restaurant & Pizzeria in South Deerfield under new ownership
 Patrons can ‘walk down memory lane’ at Sweet Phoenix’s new Greenfield location
Patrons can ‘walk down memory lane’ at Sweet Phoenix’s new Greenfield location
Arts & Life

Hitting the ceramic circuit: Asparagus Valley Pottery Trail turns 20 years old, April 27-28
A lot can change in 20 years: Presidents and other politicians come and go, new cultural fads and technologies emerge, clothing styles morph, and music and movies take on different dimensions.In these parts, one tradition hasn’t changed. Since 2005,...
Obituaries
 Diane H. Overstreet
Diane H. Overstreet
Greenfield, MA - Diane H. Overstreet (Glazier) of Greenfield succumbed to a short battle with cancer on Wednesday March 27th, 2024, at the age of 75. Born June 2nd, 1948, in Greenfield, Mas... remainder of obit for Diane H. Overstreet
 Beverly K. Fenning
Beverly K. Fenning
Greenfield, MA - Beverly Kay (Askew) Fenning, aged 82, passed into the loving arms of God on Saturday, March 23, 2024. Beverly was born in Morgantown, West Virginia, to Joshua Herman Askew... remainder of obit for Beverly K. Fenning
 Dorothy Howes
Dorothy Howes
Greenfield, MA - Dorothy Ruth "Dottie" (Tacy) Howes, 97, of Greenfield, formerly of Leyden died Tuesday, April 16, 2024, at Charlene Manor. Born in Winchester, NH, May 9, 1926, she was ... remainder of obit for Dorothy Howes
 Mark A. Matusz
Mark A. Matusz
Turners Falls, MA - Mark A. Matusz, 65, of Willmark Ave died Tuesday 4/16/24 at home surrounded by his family. He was born on August 26, 1958, the son of Thomas and Shirley (Sopellec) Matus... remainder of obit for Mark A. Matusz

 Connecting the Dots: In what world do you want to live?
Connecting the Dots: In what world do you want to live?
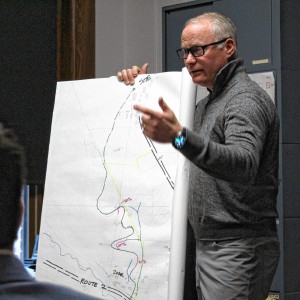 ‘Glamping’ resort developers look to curb traffic, sewer concerns in Charlemont
‘Glamping’ resort developers look to curb traffic, sewer concerns in Charlemont
 MassDOT shares plans for Church Street bridge replacement in Erving
MassDOT shares plans for Church Street bridge replacement in Erving
 Proposed Colrain solar bylaw changes would add definitions, expand site plan reviews
Proposed Colrain solar bylaw changes would add definitions, expand site plan reviews
 Deerfield voters to decide 8 capital projects at Town Meeting
Deerfield voters to decide 8 capital projects at Town Meeting
 Shelter money fading, but ‘not at the end of the line’
Shelter money fading, but ‘not at the end of the line’
 Softball: Franklin Tech’s Hannah Gilbert holds Hopkins to two hits, leads Eagles to 7-1 victory (PHOTOS)
Softball: Franklin Tech’s Hannah Gilbert holds Hopkins to two hits, leads Eagles to 7-1 victory (PHOTOS) Keeping Score with Chip Ainsworth: What’s ahead for Greg Carvel’s crew?
Keeping Score with Chip Ainsworth: What’s ahead for Greg Carvel’s crew? High schools: Abigail Schreiber’s hit propels Frontier softball past Greenfield, 3-2
High schools: Abigail Schreiber’s hit propels Frontier softball past Greenfield, 3-2 Baseball: Frontier handles Greenfield 12-2 in five-inning victory (PHOTOS)
Baseball: Frontier handles Greenfield 12-2 in five-inning victory (PHOTOS) Mitch Speight and Joan Marie Jackson: City should follow constitutional ruling on property takings
Mitch Speight and Joan Marie Jackson: City should follow constitutional ruling on property takings Valley Bounty: Your soil will thank you: As garden season gets underway, Whately farm provides ‘black gold’ to many
Valley Bounty: Your soil will thank you: As garden season gets underway, Whately farm provides ‘black gold’ to many Earth Matters: From Big Sits to Birdathons: Birding competitions far and near
Earth Matters: From Big Sits to Birdathons: Birding competitions far and near Sounds Local: Spring is singer-songwriter season: A host of local performers celebrate new work
Sounds Local: Spring is singer-songwriter season: A host of local performers celebrate new work Crunch time for matzo: An easy-to-make sweet treat that’s Passover Seder-friendly
Crunch time for matzo: An easy-to-make sweet treat that’s Passover Seder-friendly
